I mentioned that my daughter was home. I wanted to show her the grape plasma, bit it did not work because the grapes were a little ripe and just split immediately upon heating. It took some convincing, but my wife allowed a quick and simple creation of a much more impressive plasma display. If my daughter were not home, she would probably have not agreed. So thanks for that!
Let's go straight to the video because it is impressive:
We have created much more plasma that the grapes did! I have a lit match on a watch glass, held in the air by a toothpick (so it did not go out immediately), contained in a beaker. Then I pressed go on high and filmed. The plasma jumps around inside the beaker as the microwave goes through hot and cold spots of radiation. Make sure the volume is on as this makes an impressive hum.
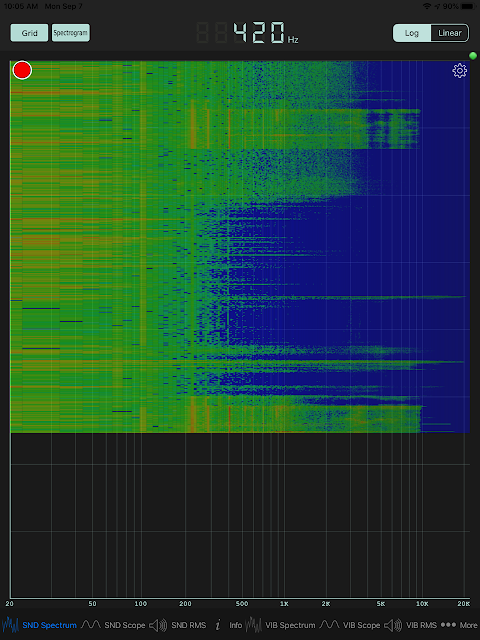
I analyzed the audio from the plasma and it seems to be regenerating exactly 420 times per second. I had expected 120 time per second based on NileRed and some other internet content. Yes, 120Hz is the frequency of rectified AC power but I don't think that matters. The microwaves themselves are in the gigahertz range. It seems to me that the principle of the microwave is that hot spots will form standing waves of electromagnetic energy according to the resonant frequency of the mechanical chamber itself. Perhaps that is around 420Hz. What really surprised me was how truly consistent the frequency (to the Hertz). See this analysis for one:
It is wonderful that I can analyze the audio with a free app on my smartphone. Ten years ago I would need a lab full of equipment for much of what I do on my phone now. The number at the top is the peak. Every time I play the video I get audio playback up to 10KHz. That number may just be a cutoff given the sampling frequency of my video. I'll skip the math and physics lecture here. The point is that the dark red line is the peak signal during the plasma generation. It is a pretty narrow and strong band of sound. Two video playbacks are shown above, with ambient noise in between. For whatever reason, I get very little sound at 120Hz and a clear and consistent peak corresponding with the plasma generation at 420Hz.
Luckily I cut the experiment pretty short. I saw no real purpose in keeping my plasma alive for multiple rotations. I did not want to risk fire or any other damage. The plasma can easily reach 10,000 degrees F and much, much higher. Let's just say that my breaker cracked from the heat in only a few seconds, and it should be good up to 1000 F or so. I often use a blow torch directly on the special glass at that temperature. I don't recommend it, just like I can't recommend this experiment for others. If my beaker had fallen apart instead of just cracking, the plasma would have been free to roam the microwave and melt anything it came near. The fire risk was probably low because I had my hand on the off button, but microwaves are built out of materials that quickly melt at these temperatures. NileRed demonstrated this, so I don't need to.
To paraphrase a baseball announcer, my beaker died a hero. My microwave still works and I am still married.
Now, what was the gas that we heated to the fourth state of matter?
Old matchheads (not safety matches were made of mostly white phosphorus). I, however, grabbed whatever free book of safety matches was available in the drawer next to the microwave. I didn't really plan this experiment, per se. I did the whole thing in just a few minutes. Wikipedia says safety matches are mostly potassium chlorate. Perhaps I'll do a whole experiment on match heads now. Chemistry begats more chemistry. I figure there was a fair bit of air that was ionized as well as some carbon gasses from the paper of the match.
Whatever, I had neutral gasses from the flame, and I zapped it to kingdom come with a strong electric field (apparently 420 times per second). I ripped the electrons off of potassium ions and/or carbon ions and/or whatever else there was in air, including Nitrogen, to create a free cloud of electrons that emitted yellowish photons in the form of light energy. Most likely the dominant yellow color is from sodium ions that came from sodium borosilicate beaker glass. Shout out to NileRed for doing some good experiments to indicate this.
If you saw his video, I was thinking the beaker glass was causing his Na spike while he went super-technical in search of chemoluminescent reactions and so forth. I don't know 1% of what he does, so I figured that beaker glass is somewhat related to soda-lime glass. He knew enough to seek far more complicated theories before determining the simpler truth. When you are at this high a temperature, all of the normal rules don't necessarily apply. Your beaker may not be inert. Super strong Nitrogen triple bonds break and this mostly "inert" gas reacts with oxygen to form NO2. Weird, wild stuff.
I declare success and a truce in the plasma war before things become too heated.
Thanks for reading,
Paul



No comments:
Post a Comment